R = 8.314 J mol-1 K-1 = 0.08206 L atmK-1 mol-1 = 0.08314 L bar K-1 mol-1 NA = 6.022 x 1023 mol-1 kB = 1.381 x 10-23 J K-1 h = 6.626 x 10-34 J s F = 96,500 C mol-1 c = 2.998 x 108 m s-1 g = 9.81 m s-2 B = 0.51mol-1/2dm3/2 (in H2O, 25oC) Sequential reactions: [B]=(k1/(k2-k-1)) f(t)[A]0 f(t)=exp(-k1t)-exp(-k2t) Parallel reactions: Φi = ki/S Where S is the sum of all rate constants of the paral-lel reactions Note: Quantum yield/efficiency = Φ = moles of product formed / moles of photons absorbed | 1dm3 = 1 L 1dm3 = 1000 cm3 1 J = 1 kg m2 s-2 1 atm = 1.101325 x 105 Pa 1 atm = 760 mmHg 1 Torr = 1 mmHg 1 Torr = 133.322 Pa 1 bar = 105 Pa 1 nm = 10-9 m Eyring equation: k =kBT/(hc0) x f f =exp(ΔS#/R) x x exp(-ΔH#/RT) ln(1 - θ) = -θ if θ << 1 | E = hυ c =υλ PV =nRT ΔG = ΔH - TΔS k = A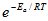 k = 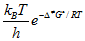 A = e (kBT/h) exp (Δ‡S/R)
k = 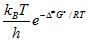 Ea = Δ≠Ho-PΔ≠Vo + RT (sol) = Δ≠Ho-ΣνRT + RT (gas) log k = log ko+ 1.02 zAzB 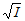 log k = log ko - 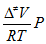 ΔGo = - RT ln Kc υ = 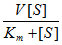 θ = KP/(1 + KP), at T=const tf = (kf +kq[Q])-1, Q is quencher |