Figure 1
SUMMARY
OF MASTER THESIS PROJECT
1,2-Additions
to Activated Imines
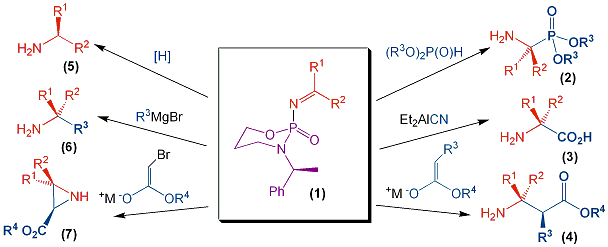
There are no literature reports of imines activated by chiral phosphorus auxiliaries. Thus, we set out to develop suitable methodology for the synthesis of a novel class of enantiomerically pure imine (1). These are potential precursors to many biologically important building blocks such as α-aminophosphonic acids (2), α-amino acids (3), β-amino acids (4), α-branched amines (5), α,α-dibranched amines (6) and aziridines (7) (figure 1).

Figure
1
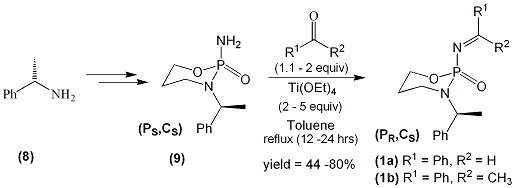
We recently developed a synthetic route to this class of imines (1). Starting from the commercially available (S)-α-methylbenzylamine (8) the precursor phosphorodiamidate (9) was synthesized in four steps. This included separation of a diastereomeric mixture of phosphoramidochloridates by column chromatography in order to yield the desired homochiral substrates. X-Ray analysis of phosphorodiamidate (9) allowed for the assignment of the absolute stereochemistry of this substrate. Following considerable experimentation suitable conditions were found for synthesis of both the corresponding aldimines (1a) and the more synthetically challenging ketimines (1b) (scheme 1) [1]. We modified conditions employed by Davis and Ellman for the synthesis of enantiomerically pure sulfinimines [2,3].
Scheme
1
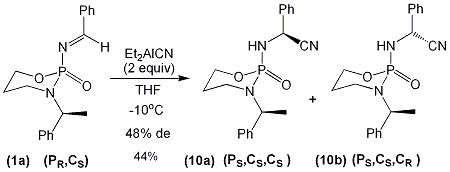
Our preliminarily 1,2-addition reactions of cyanide nucleophiles to the benzaldehyde derived imine (1a) proceeded with moderate levels of diastereoseletivity (up to 48% de) (scheme 2) [1]. The major addition product was obtained in a diastereomerically pure form, following recrystallization of the diastereomeric addition products (10a) and (10b). We intend to utilize X-Ray analysis in order to determine the absolute stereochemistry of our major addition product, and thus shed light upon the stereochemical outcome of this reaction.
Scheme
2
Recently, we have developed a “one-pot” variant of our imine formation / nucleophilic addition protocol. Thus, addition of the nucleophile is now possible without prior purification of the imine. In future we hope to extend this methodology for the synthesis of other classes of aldimines and ketimines including aliphatic, and other enolizable derivatives, as well as those that are potential precursors to both natural and unnatural α-aminoacids.
[1]
N. Abu-Thabit, I. Forristal, unpublished results, Masters Thesis, KFUPM, May 2005.
[2] P. Zhou, B. C. Chen, F. A. Davis, Tetrahedron, 2004,
60, 8003.
[3] J. A. Ellman, T. D. Owens, T. P. Tang, Acc. Chem. Res., 2002,
35, 984.
SUMMARY OF MY PhD PROJECT :